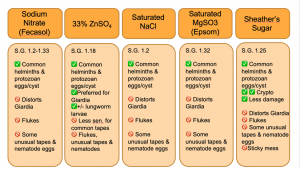
Fecal flotation is by far the most common diagnostic procedure used to detect intestinal parasites in our veterinary species due to its low cost and is a relatively straightforward procedure to conduct. The majority of parasite ova/ eggs have a specific gravity (SG) that falls between 1.05 and 1.23, thus the fecal flotation relies on the ascension of eggs and ova using the difference in overall density to the flotation solution. 1,2 The most common flotation solutions used in veterinary medicine include saturated sodium chloride, sodium nitrate, sugar (e.g. Sheather’s solution), magnesium sulfate, and zinc sulfate. In the perfect scenario, a fecal flotation solution would preserve egg and oocyst morphologic integrity while limiting the ascension of fecal debris; however, not all flotation solutions are created equal. For example, sugar solutions have an SPG of 1.25 resulting in Giardia cysts becoming distorted and difficult to identify for the novice, but a better overall ova yield than other solutions. In addition to choosing the best flotation solution for the parasites, a practitioner is wishing to recover, passive versus centrifugal flotation methods can yield vastly different results. In general, centrifugal flotation techniques are considered more sensitive in recovering parasite ova.
Centrifugation methods use centripetal motion to aid in the suspension of the helminths eggs and protozoan oocysts in a solution versus passive relying completely on the density and specific gravity for ascension. 3 The S.G. of flotation solutions should be checked periodically, at least monthly, or when opening a new bottle. You can easily check the S.G. of your flotation in your clinic using a hydrometer.

In today’s laboratory, you will practice both centrifugal and passive flotation techniques.
A few questions to ask yourself as you practice these two techniques are the following:
The following instructions are for the technique we will be using in the laboratory sessions.
Fecalyzer ® is a commercial kit that is widely used in primary care settings. The technique below describes the passive technique without the kit. In the laboratory, we will practice using the Fecalyzer ® kit to recover parasite ova using the kit’s instructions. Fecalyzer ® kits use a commercially available flotation solution, Fecasol ®. It is a NaNO3 flotation solution with SpG 1.20 (not saturated) and will float most common eggs and oocysts, but will distort Giardia cysts. In the laboratory today, we will use ZnSO4 instead of NaNO3.
Video: This video demonstrates the passive fecal flotation technique using a Fecalyzer ® kit. There are other methods for performing the passive fecal location that is outlined in your parasitology laboratory manual.
The following instructions are for the technique we will be using in the laboratory sessions.
The centrifugal flotation technique you will practice in the laboratory is a two-step process with the first step being a “wash” to help decrease the amount of fecal debris. In the clinical laboratory setting, people often skip the water “wash”, but in this laboratory, since it is assumed that you are still trying to learn the difference between plant or fecal debris and parasites, we will perform the water wash.
*A drop of Lugol’s iodine can be placed on the slide before placing the coverslip to enhance the identification of Giardia cysts (we will not do this in the lab).
Video: This video demonstrates the passive centrifugal flotation technique. This method is also outlined in your parasitology laboratory manual.